One number is carbon's element number or atomic number. Atomic number increase as you go across the table. This is not the value you want. The atomic mass or atomic weight is the decimal number, The number of significant figures varies according to the table, but the value is around The atomic mass of carbon would be To calculate the atomic mass of a single atom of an element, add up the mass of protons and neutrons.
Example: Find the atomic mass of an isotope of carbon that has 7 neutrons. You can see from the periodic table that carbon has an atomic number of 6, which is its number of protons. The atomic mass of an element is a weighted average of all the element's isotopes based on their natural abundance. It is simple to calculate the atomic mass of an element with these steps. Typically, in these problems, you are provided with a list of isotopes with their mass and their natural abundance either as a decimal or percent value.
The answer is the total atomic mass or atomic weight of the element. What is the relative atomic mass of the element?
Relative atomic mass
First, convert the percentages to decimal values by dividing each percentage by The sample becomes 0. Tip: You can check your math by making certain the decimals add up to 1.
- massachusetts health insurance rights for divorced spouses.
- Test your vocabulary with our fun image quizzes;
- Connect with us;
- Learn more about Relative Atomic Mass;
- birth certificate form jersey new.
Advanced Note: This atomic mass is slightly higher than the value given in the periodic table for the element carbon. What does this tell you?
Search Site Content
The sample you were given to analyze contained more carbon than average. You know this because your relative atomic mass is higher than the periodic table value , even though the periodic table number includes heavier isotopes, such as carbon Over time, you may notice the atomic mass values listed for each element on the periodic table may change slightly. This happens when scientists revise the estimated isotope ratio in the crust. In modern periodic tables, sometimes a range of values is cited rather than a single atomic mass. Find More Worked Examples. What is the standard mass unit?
Relative atomic mass is explained below, with reference to the carbon atomic mass scale and the relevance of isotopes and 'u' the unified atomic mass unit is explained. Detailed examples of the method of how to calculate relative atomic mass from the isotopic composition are fully explained with reference to the definition of the relative atomic mass of a compound. For A level students, how to define and use relative isotopic masses to calculate relative atomic mass.
- Atomic structure;
- Periodic Table of Elements with Relative Atomic Masses.
- relative atomic mass.
- Periodic Table of Elements with Relative Atomic Masses.
- Introduction;
Spotted any careless error on my part? EMAIL query? Self-assessment Quizzes on relative atomic mass:. Revision notes on how to define relative atomic mass and how to calculate relative atomic mass from the percentage abundance of isotopes, help in revising for A level AQA, Edexcel, OCR 21st century, Gateway science GCSE chemistry examinations 1. Explaining and how to calculate the relative atomic mass RAM or A r of an element.
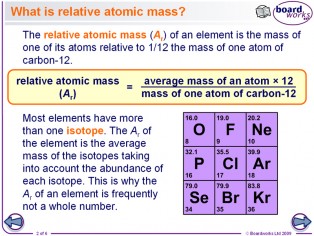
What is the relative atomic mass of an element? What scale is relative atomic mass based on? What is the formula to work out the relative atomic mass of an element? How to calculate relative atomic mass. Above is typical periodic table used in GCSE science-chemistry specifications.
- dickson county fl tax records assessor.
- How do you find relative atomic mass? + Example.
- babie death search for free.
- Atomic Weights and Isotopic Compositions with Relative Atomic Masses | NIST.
- Related terms:;
Revision notes on how to define relative atomic mass and how to calculate relative atomic mass from the percentage abundance of isotopes, help in revising for A level AQA, Edexcel, OCR 21st century, Gateway science GCSE chemistry examinations. How do I calculate relative atomic mass? You can calculate relative atomic mass from isotopic abundances.
How to calculate relative atomic mass with accurate relative isotopic masses.
Using data from modern very accurate mass spectrometers. This definition of relative isotopic mass is a completely different from the definition of relative atomic mass, except both are based on the same international standard of atomic mass i. If we were to redo the calculation of the relative atomic mass of chlorine example 1.
It is possible to do the reverse of a relative atomic mass calculation if you know the A r and which isotopes are present. The A r of boron is The relative atomic mass of boron was obtained accurately in the past from chemical analysis of reacting masses but now mass spectrometers can sort out all of the isotopes present and their relative abundance. Self-assessment Quizzes on relative atomic mass. A typical periodic table used in pre-university examinations.
Relative atomic mass and relative formula mass
Above is typical periodic table used in GCSE science-chemistry specifications in doing chemical calculations, and I've 'usually' used these values in my exemplar calculations to cover most syllabuses. Table of relative atomic masses for elements 1 to AND all their isotopes are highly radioactive and most are very unstable so your relative atomic mass changes all the time! Doc Brown's Chemistry. Enter chemistry words e.
Relative atomic mass and formula mass find mass number
STUDY notes on page or enter key words or use above links. Chemical Symbol Element name Atomic No. Z Relative atomic mass Ac Actinium 89 Z Relative atomic mass Mo Molybdenum 42